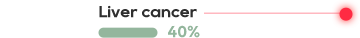IIT & IND in Q1 2023
IIT & IND in Q1 2023
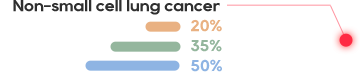
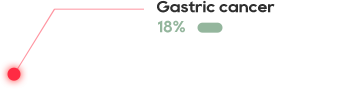
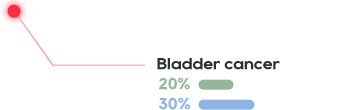
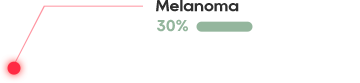
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
NY-ESO-1 is typically associated with poor prognosis, with expression often in advanced metastatic tumors.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
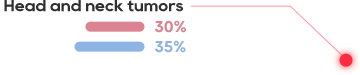
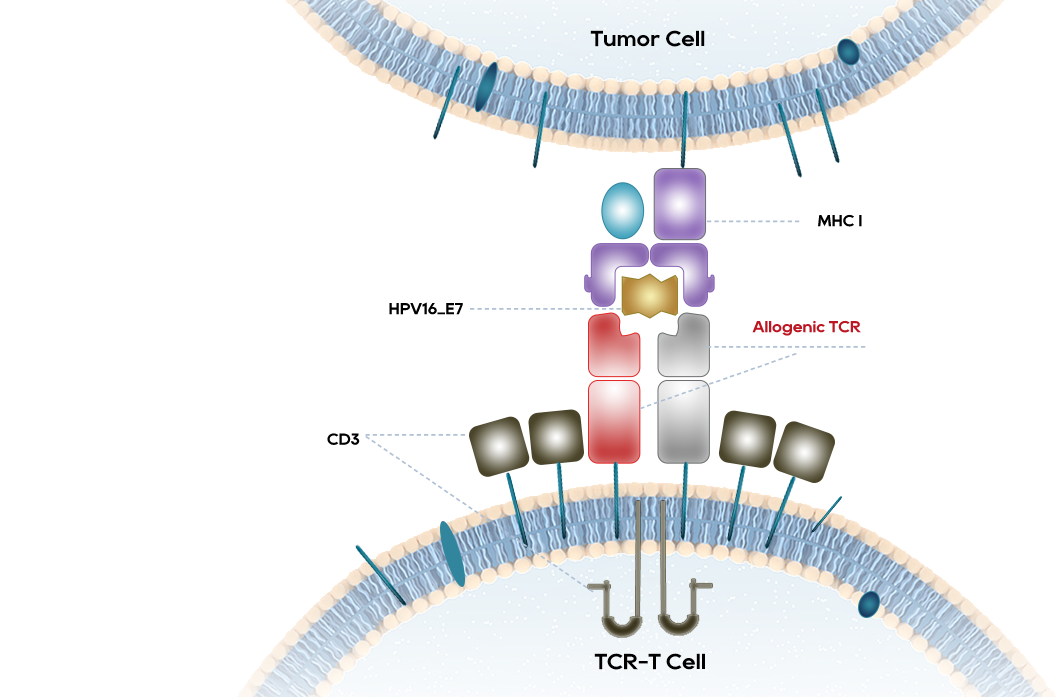
The best-in-class TCR-T therapy in China for the treatment of HPV16 related advanced cervical cancer, anal cancer and head and neck tumors
The HPV prevalence rate in Chinese women reaches 21.1%, and causes 110000 new cases and 60000 deaths of cervical cancer per year.
Nearly 40% of Chinese women cannot be vaccinated against HPV due to HPV positive or over age
Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease, after receiving TCR-T targeting HPV-16 E7.
Approved IIT ethics will be started soon
ND declaration at the end of 2023
Approved IIT ethics will be started soon
ND declaration at the end of 2023
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
NY-ESO-1 is typically associated with poor prognosis, with expression often in advanced metastatic tumors.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
The best-in-class TCR-T therapy in China for the treatment of HPV16 related advanced cervical cancer, anal cancer and head and neck tumors
The HPV prevalence rate in Chinese women reaches 21.1%, and causes 110000 new cases and 60000 deaths of cervical cancer per year.
Nearly 40% of Chinese women cannot be vaccinated against HPV due to HPV positive or over age
Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease, after receiving TCR-T targeting HPV-16 E7.
Q3 IIT in 2022 IND declaration at the beginning of 2024
Q3 IIT in 2022 IND declaration at the beginning of 2024
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
NY-ESO-1 is typically associated with poor prognosis, with expression often in advanced metastatic tumors.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
The best-in-class TCR-T therapy in China for the treatment of HPV16 related advanced cervical cancer, anal cancer and head and neck tumors
The HPV prevalence rate in Chinese women reaches 21.1%, and causes 110000 new cases and 60000 deaths of cervical cancer per year.
Nearly 40% of Chinese women cannot be vaccinated against HPV due to HPV positive or over age
Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease, after receiving TCR-T targeting HPV-16 E7.
Q3 IIT in 2022
IND declaration at the beginning of 2024
Q3 IIT in 2022
IND declaration at the beginning of 2024
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
NY-ESO-1 is typically associated with poor prognosis, with expression often in advanced metastatic tumors.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
The best-in-class TCR-T therapy in China for the treatment of HPV16 related advanced cervical cancer, anal cancer and head and neck tumors
The HPV prevalence rate in Chinese women reaches 21.1%, and causes 110000 new cases and 60000 deaths of cervical cancer per year.
Nearly 40% of Chinese women cannot be vaccinated against HPV due to HPV positive or over age
Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease, after receiving TCR-T targeting HPV-16 E7.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
NY-ESO-1 is typically associated with poor prognosis, with expression often in advanced metastatic tumors.
The cell surface antigen New York esophageal squamos cell carcinoma 1 (NY-ESO-1) is a clinically validated target present in many aggressive solid tumors including synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCLS) as well as non-small cell lung cancer (NSCLC), bladder cancer, melanoma and liver cancer.
The best-in-class TCR-T therapy in China for the treatment of HPV16 related advanced cervical cancer, anal cancer and head and neck tumors
The HPV prevalence rate in Chinese women reaches 21.1%, and causes 110000 new cases and 60000 deaths of cervical cancer per year.
Nearly 40% of Chinese women cannot be vaccinated against HPV due to HPV positive or over age
Robust tumor regression was observed with objective clinical responses in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease, after receiving TCR-T targeting HPV-16 E7.