IND in 2024
IND in 2024
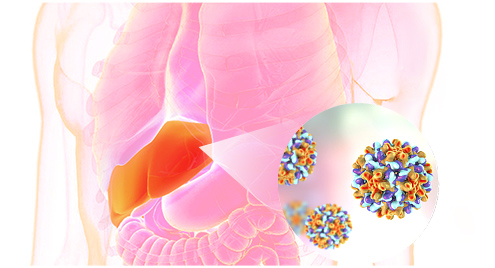
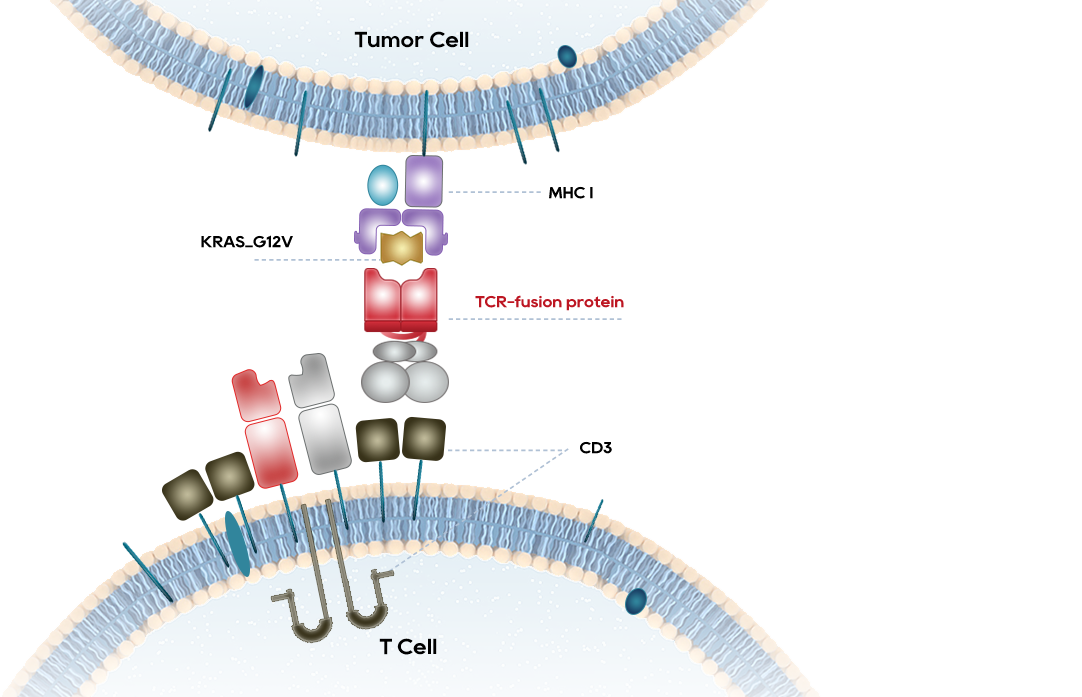
The world's first TCR-T therapy for the treatment of advanced solid tumors with KRAS G12V mutation.
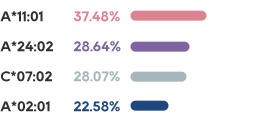
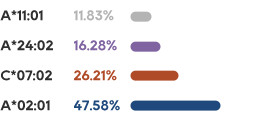
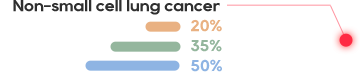
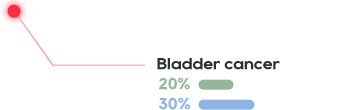
KRAS is one of the most common mutations in solid tumors, which exists in about 90% of pancreatic cancer, 40% of colorectal cancer and 30% of lung adenocarcinoma.
The vast majority of patients acquired resistance to G12C inhibitors in a short time, and no available candidate drugs for G12D and G12V.
KRAS specific TCR-T have showed significant curative effect in both colorectal cancer and pancreatic cancer.
IND in 2024
IND in 2024
The world's first TCR-T therapy for the treatment of advanced solid tumors with KRAS G12V mutation.
KRAS is one of the most common mutations in solid tumors, which exists in about 90% of pancreatic cancer, 40% of colorectal cancer and 30% of lung adenocarcinoma.
The vast majority of patients acquired resistance to G12C inhibitors in a short time, and no available candidate drugs for G12D and G12V.
KRAS specific TCR-T have showed significant curative effect in both colorectal cancer and pancreatic cancer.
The world's first TCR-T therapy for the treatment of advanced solid tumors with KRAS G12V mutation.
KRAS is one of the most common mutations in solid tumors, which exists in about 90% of pancreatic cancer, 40% of colorectal cancer and 30% of lung adenocarcinoma.
The vast majority of patients acquired resistance to G12C inhibitors in a short time, and no available candidate drugs for G12D and G12V.
KRAS specific TCR-T have showed significant curative effect in both colorectal cancer and pancreatic cancer.
The world's first TCR-T therapy for the treatment of advanced solid tumors with KRAS G12V mutation.
KRAS is one of the most common mutations in solid tumors, which exists in about 90% of pancreatic cancer, 40% of colorectal cancer and 30% of lung adenocarcinoma.
The vast majority of patients acquired resistance to G12C inhibitors in a short time, and no available candidate drugs for G12D and G12V.
KRAS specific TCR-T have showed significant curative effect in both colorectal cancer and pancreatic cancer.
The world's first TCR-T therapy for the treatment of advanced solid tumors with KRAS G12V mutation.
KRAS is one of the most common mutations in solid tumors, which exists in about 90% of pancreatic cancer, 40% of colorectal cancer and 30% of lung adenocarcinoma.
The vast majority of patients acquired resistance to G12C inhibitors in a short time, and no available candidate drugs for G12D and G12V.
KRAS specific TCR-T have showed significant curative effect in both colorectal cancer and pancreatic cancer.
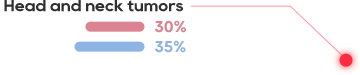
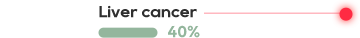







•About 300million patients worldwide, tens of millions of patien
•There is no effective treatment
•HPV specific TCR-fusion protein therapy is expected to eliminate HPV infected cells and cure HPV infection
•About 260million patients worldwide and 70 million patients in China
•Available treatment can only inhibit virus replication, but can't eliminate the virus
•HBV specific TCR-fusion protein therapy is expected to eliminate infection