On November 21, 2023, CorreGene's groundbreaking cell therapy product, CRTE7A2-01 TCR-T, has received the NMPA official approval for clinical trials.


Date:2023-11-21 19:33:00 Browse:
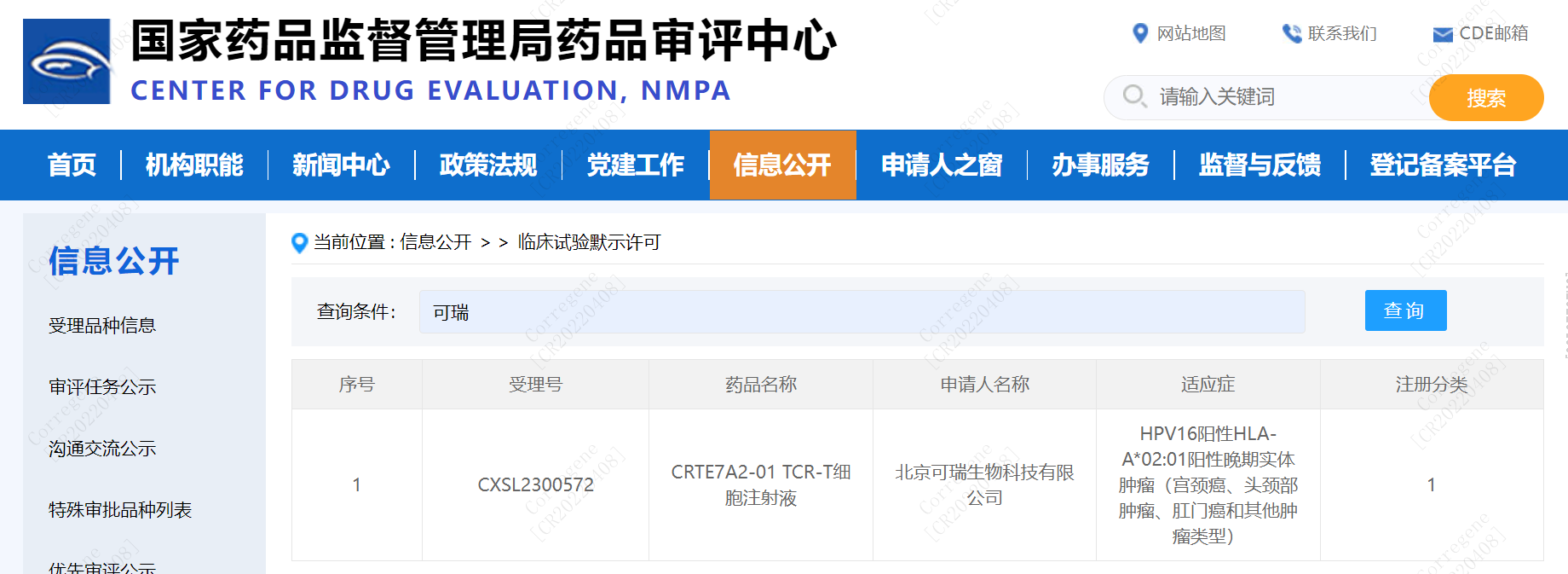
The indications for CRTE7A2-01 TCR-T cell therapy product are HPV16-positive HLA-A*02:01-positive advanced solid tumors, including cervical cancer, head and neck tumors, anal cancer, and other tumors. On November 21, 2023, after successfully addressing all concerns raised by the Center for Drug Evaluation (CDE) during the Pre-IND communication meeting and IND review, CorreGene received the clinical trial approval notification for CRTE7A2-01 TCR-T. Simultaneously, the CDE official website published the approval for the clinical trial of CorreGene's CRTE7A2-01 TCR-T cell injection. This marks a significant milestone for CorreGene as we embark on the next phase of advancing towards registered clinical trials.