IND in 2024
IND in 2024
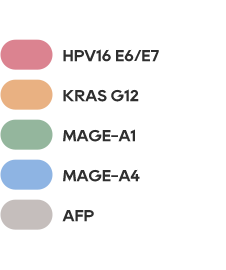
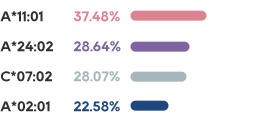
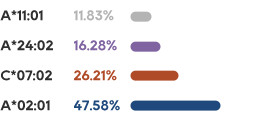
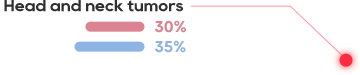
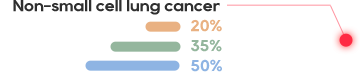
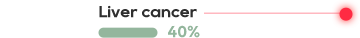



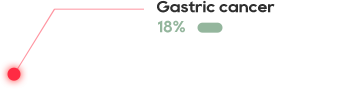


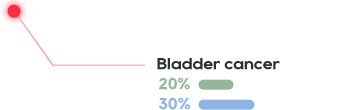


•There has not been an effective treatment yet for the about 300 million patients worldwide, with tens of millions of patients in China
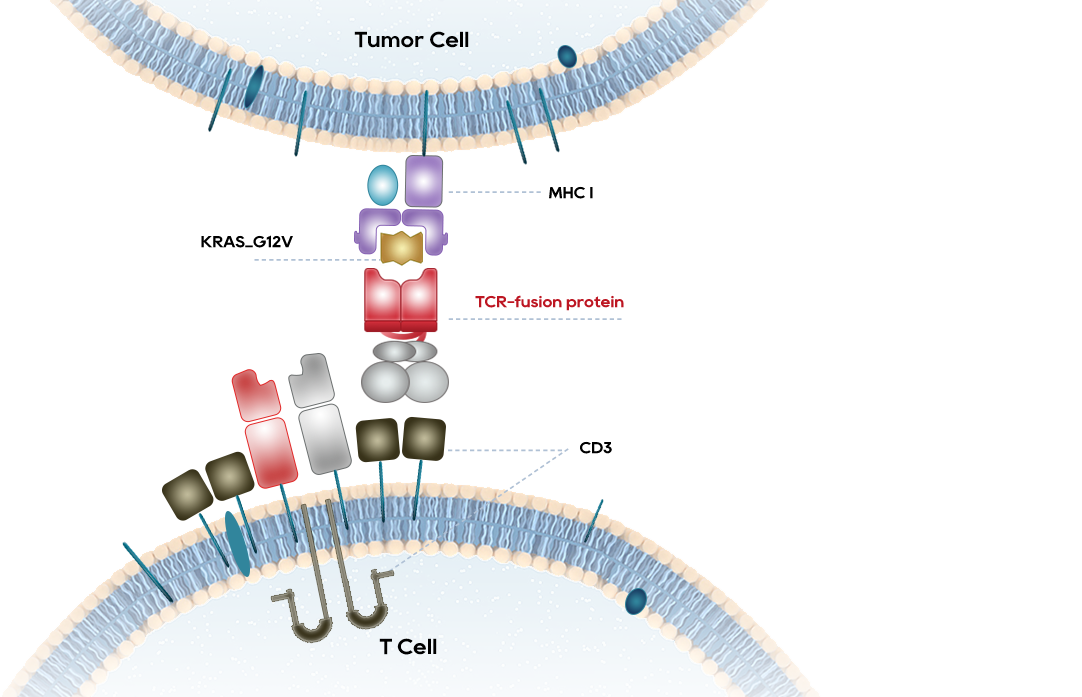
•HPV specific TCR bispecific antibody are expected to (clear infections)eliminate cells infected by HPV and cure HPV infection
•Approximately 260 million patients worldwide, with 70 million in China
•Currently, the virus can only be inhibited to replicate but not eliminated
•HBV specific TCR bispecific antibody are expected to clear infections