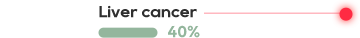IIT & IND in H1 2023
IIT & IND in H1 2023
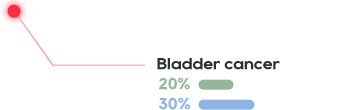
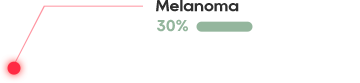
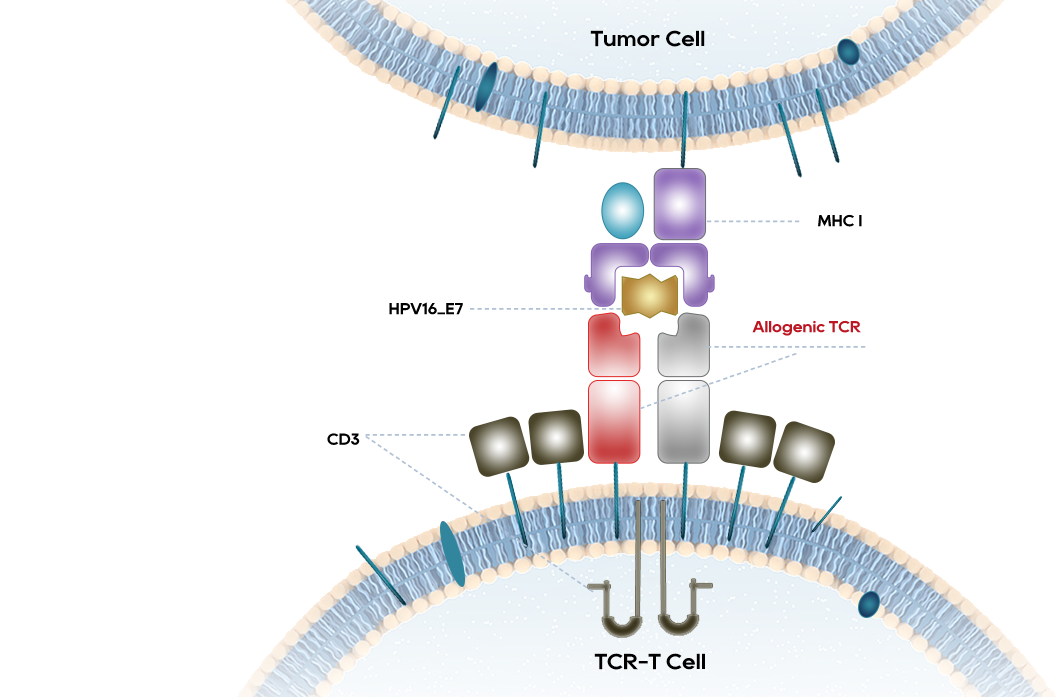
CRTE7A2-01 treatment for HPV-positive people
The pioneering TCR-T cell therapy in China to treat advanced HPV16 positive cervical cancer, anal cancer, and head and neck cancer
The HPV infection rate among women in China reached 21.1%, and as a result, there are 110,000 new cases of cervical cancer per year and 60,000 deaths
Nearly 40% of women in China cannot vaccinate because of HPV positive or being overage
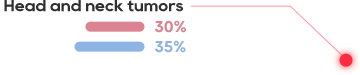
HPV TCR-T is effective in the treatment of HPV-positive cervical cancer, head and neck squamous cell carcinoma, 6/12 cases showed significant shrinkage
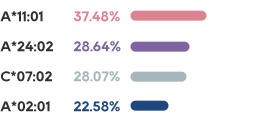
IIT in H1 2023
IIT in H1 2023
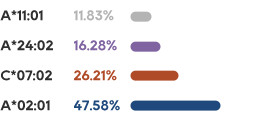
IIT in H1 2022
IIT in H1 2022
TCR-T CRTKVA11-01 treatment for people with KRAS mutation
Globally innovative TCR-T cell therapy for treating advanced solid tumors with KRAS G12V mutation
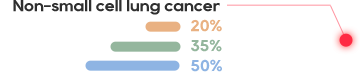
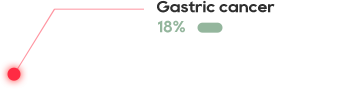
KRAS is one of the most common mutations in solid tumors, and is present in about 90% pancreatic cancer, 40% colorectal cancer, and 30% lung adenocarcinoma
G12C inhibitors have a short duration of efficacy, and there are no available candidate drugs for G12D and G12V.
Case reports of KRAS TCR-T therapy can completely clear metastases and maintain 4 years of disease-free survival (DFS)
TCR-T CRTKVA11-01 treatment for people with KRAS mutation
Globally innovative TCR-T cell therapy for treating advanced solid tumors with KRAS G12D mutation
KRAS is one of the most common mutations in solid tumors, and is present in about 90% pancreatic cancer, 40% colorectal cancer, and 30% lung adenocarcinoma
G12C inhibitors have a short duration of efficacy, and there are no available candidate drugs for G12D and G12V.
Case reports of KRAS TCR-T therapy can completely clear metastases and maintain 4 years of disease-free survival (DFS)